Thermodynamic Database
Components (31)
Total of 31 components are included in the database as listed here:
Ag-Al-Bi-C-Ca-Ce-Co-Cu-Dy-Er-Fe-Gd-La-Li-Mg-Mn-Nd-Ni-O-P-Pr-Sb-Sc-Se-Si-Sm-Sn-Sr-Y-Zn-Zr
Suggested Composition Range
The suggested composition range for each element is listed in Table 1. It should be noted that this given composition range is rather conservative. It is derived from the chemistries of the multicomponent commercial alloys that have been used to validate the current database. In the subsystems, many of these elements can be applied to a much wider composition range. In fact, some subsystems are valid in the entire composition range as given in Section Thermodynamic Database.
There is no composition limit for most binary and many ternary systems formed with major components. These subsystems are valid in the full composition range from 0-100% as detailed in ~ below. Oxygen containing systems are generally restricted to solid oxides and temperature below 1000°C, only the Mg-Ca-O and Si-O systems include the liquid oxide.
Phases
Total of 834 phases are included in the database. The phase names are selected according to the following strict rules in Table 2 for consistent and intuitive phase identification in different categories. In the category of solid solution phases, indicated in the TDB Viewer of Pandat™ as CEF(SLN), we distinguish
SE = Solution phase extending to at least one stable phase of an elemental component, disordered.
SI = Solution intermetallic phase usually described by a model with more than one sublattice.
In the category of stoichiometric phases (fixed composition) the TDB Viewer of Pandat displays CEF(ST*), where * indicates the number of components:
ST1 = Unary stoichiometric phase
ST2 = Binary stoichiometric phase
ST3 = Ternary stoichiometric phase
ST4 = Quaternary stoichiometric phase.
*) Rare earth elements do not include Sc and Y; Lanthanides only R = (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er,Tm, Yb, Lu).
**) Non-metals are from "Pettifor-string", chemical order from 88 to 102, (Sb, As, P, Te, Se, S, C, I, Br, Cl, N, O, F), and listed according to the periodic table.
The total 834 phases assessed in PanMg2024 comprise the 234 solution phases (gas phase, liquid, plus 232 solid solution phases) and 600 stoichiometric phases. These 600 comprise 4 unary, 513 binary, 81 ternary, and 2 quaternary assessed stoichiometric phases.
As an illustration of the names and models assigned to the various solution and stoichiometric phases a small representative selection is listed in Table 3. For all phases assessed in PanMg2024 is listed in PanMg2024:List of All Phases. Users can also view them in the TDB viewer of Pandat™ software.
*For liquidus projection calculations it is usually recommended to suspend the gas phase
What is new in PanMg2024
While previous developments of PanMg were a continuous evolution from 1995 onwards the current PanMg2024 comprises a grand revision and upgrade prepared during the past three years. It includes the revised data for two elements and 23 binary systems, as well as revised or additional descriptions for 20 ternary and 4 quaternary alloy systems. In this “second generation” of PanMg the wealth of recent experimental data could only be included by undertaking the revision of core systems.
One example is the Mg-Zn core system that was completely revised based on the new and first detailed documented modeling of [2022Li]. The revised solidus of (Mg) also enabled the precise incorporation of recent experimental data on Mg-Zn-Ca (ZX) alloys [2014Hof, 2016Cao, 2022Qiu, 2022Sch] together with the data in the full composition range of the Ca-Mg-Zn system. As a consequence, all Mg-Zn-* ternary and quaternary systems needed revision and at that occasion were also completely revised incorporating also the more recent experimental data.
Another example are the data for the elements Y and Gd which were updated based on experimental data [1995She, 1996She] not included in the classic SGTE data of [1991Din]. Binary Mg-Y and Mg-Gd systems were updated based on the most recent descriptions [2020Wu1, 2020Wu2] but important adjustments are made to fit experimental data in the Mg-rich corner more precisely. Many related Mg-RE systems were also updated including recent data.
Consequently, the very complex Mg-Y-Zn and Mg-RE-Zn ternary systems underwent full re-optimization and upgrade to include the latest experimental data based on dedicated TEM studies. Some details are given in “Section Database Validation” for the most important Mg-Y-Zn and Mg-Gd-Zn systems. The description for quaternary Gd-Mg-Y-Zn alloys is also revised.
Many other binary and ternary systems were also rigorously updated including the most recent experimental data. Focus was on Mg-rich alloys but also on remote systems with impact on Mg alloys. One example is the Al-Cu-Zn system, also with new binaries, and Al-Cu-Mg-Zn quaternary alloys with all ternary subsystems assessed.
Key improvements include:
-
23 improved binary system descriptions of Al-Cu, Al-Sm, Ce-La, Ce-Nd, Ce-Y, Ce-Zn, Cu-Y, Cu-Zn, Cu-Zr, Dy-Mg, Fe-Gd, Gd-Li, Gd-Mg, Gd-Sb, Gd-Y, Gd-Zn, La-Nd, Li-Si, Mg-Y, Nd-Y, Ni-Y, Sn-Y, Y-Zr.
-
20 improved or new ternary system descriptions of Ag-Al-Cu, Ag-Mg-Sb, Al-Ce-Mn, Al-Cu-Mg, Al-Cu-Mn, Al-Cu-Zn, Al-Gd-Mn, Al-Mg-Zn, Al-Mn-Nd, Ca-Mg-Zn, Ce-Mg-Y, Ce-Mg-Zn, Cu-Mg-Zn, Cu-Y-Zr, Gd-Li-Mg, Gd-Mg-Y, Gd-Mg-Zn, La-Mg-Zn, Mg-Mn-Zn, Mg-Y-Zn.
-
Four improved or new quaternary system descriptions of Al-Ca-Mg-Mn, Al-Cu-Mg-Zn, Al-Mg-Mn-Zn, Gd-Mg-Y-Zn.
Assessed Subsystems
A total of 378 systems, including 247 binary, 114 ternary and 17 quaternary subsystems have been assessed. The modeling status is indicated by numbers. The systems with number 10 are fully assessed in the whole composition range. The higher value shows higher reliability of the system.
Binary Systems (247)
| Ag-Al(10) | Ag-Ca(10) | Ag-Ce(10) | Ag-Co(10) | Ag-Cu(10) | Ag-Er(10) | Ag-Fe(10) |
| Ag-Gd(10) | Ag-La(10) | Ag-Mg(10) | Ag-Mn(10) | Ag-Nd(5) | Ag-Ni(10) | Ag-Sb(10) |
| Ag-Sc(10) | Ag-Si(10) | Ag-Sm(10) | Ag-Sn(5) | Ag-Sr(10) | Ag-Y(5) | Ag-Zn(10) |
| Ag-Zr(5) | Al-Bi(10) | Al-C(10) | Al-Ca(10) | Al-Ce(10) | Al-Co(10) | Al-Cu(10) |
| Al-Er(10) | Al-Fe(10) | Al-Gd(10) | Al-La(10) | Al-Li(10) | Al-Mg(10) | Al-Mn(10) |
| Al-Nd(10) | Al-Ni(10) | Al-P(10) | Al-Pr(10) | Al-Sb(10) | Al-Sc(10) | Al-Si(10) |
| Al-Sm(10) | Al-Sn(10) | Al-Sr(10) | Al-Y(10) | Al-Zn(10) | Al-Zr(10) | Bi-Mg(10) |
| Bi-O(5) | Bi-Sb(10) | Bi-Sn(10) | Bi-Zn(10) | C-Ca(5) | C-Li(10) | C-Mg(10) |
| C-Si(10) | C-Sn(10) | Ca-Ce(5) | Ca-Cu(10) | Ca-Fe(10) | Ca-Gd(5) | Ca-Li(10) |
| Ca-Mg(10) | Ca-Mn(10) | Ca-Nd(5) | Ca-Ni(5) | Ca-O(10) | Ca-P(5) | Ca-Sb(10) |
| Ca-Sc(10) | Ca-Si(10) | Ca-Sn(10) | Ca-Sr(10) | Ca-Y(5) | Ca-Zn(10) | Ca-Zr(5) |
| Ce-Co(10) | Ce-Cu(10) | Ce-Fe(10) | Ce-La(10) | Ce-Li(5) | Ce-Mg(10) | Ce-Mn(5) |
| Ce-Nd(10) | Ce-Ni(10) | Ce-Sb(10) | Ce-Sc(5) | Ce-Si(5) | Ce-Sr(5) | Ce-Y(10) |
| Ce-Zn(10) | Ce-Zr(5) | Co-Cu(10) | Co-Er(10) | Co-Fe(5) | Co-Gd(10) | Co-La(10) |
| Co-Mg(10) | Co-Mn(10) | Co-Nd(10) | Co-Ni(10) | Co-Pr(10) | Co-Sb(10) | Co-Sc(5) |
| Co-Si(10) | Co-Sm(10) | Co-Sn(10) | Co-Y(10) | Co-Zn(10) | Co-Zr(10) | Cu-Er(10) |
| Cu-Fe(10) | Cu-Gd(10) | Cu-La(10) | Cu-Li(5) | Cu-Mg(10) | Cu-Mn(10) | Cu-Nd(10) |
| Cu-Ni(10) | Cu-Sb(10) | Cu-Sc(5) | Cu-Si(10) | Cu-Sm(10) | Cu-Sn(10) | Cu-Sr(10) |
| Cu-Y(10) | Cu-Zn(10) | Cu-Zr(10) | Dy-Mg(10) | Er-Fe(10) | Er-Mg(10) | Er-Sb(10) |
| Er-Sc(10) | Er-Si(10) | Er-Y(10) | Er-Zn(10) | Fe-Gd(10) | Fe-Mg(10) | Fe-Mn(10) |
| Fe-Nd(10) | Fe-Ni(10) | Fe-Sb(10) | Fe-Sc(10) | Fe-Si(10) | Fe-Sm(10) | Fe-Sn(10) |
| Fe-Sr(10) | Fe-Y(10) | Fe-Zn(10) | Fe-Zr(10) | Gd-Li(10) | Gd-Mg(10) | Gd-Mn(10) |
| Gd-Ni(10) | Gd-Sb(10) | Gd-Sc(5) | Gd-Si(10) | Gd-Sm(10) | Gd-Y(10) | Gd-Zn(10) |
| Gd-Zr(10) | La-Mg(10) | La-Mn(10) | La-Nd(10) | La-Ni(10) | La-Sb(10) | La-Sc(5) |
| La-Si(10) | La-Sn(10) | La-Zn(10) | Li-Mg(10) | Li-Mn(5) | Li-Sc(10) | Li-Si(10) |
| Li-Sn(10) | Li-Sr(5) | Li-Y(5) | Li-Zn(5) | Li-Zr(5) | Mg-Mn(10) | Mg-Nd(10) |
| Mg-Ni(10) | Mg-O(10) | Mg-P(5) | Mg-Pr(10) | Mg-Sb(10) | Mg-Sc(10) | Mg-Se(5) |
| Mg-Si(10) | Mg-Sm(10) | Mg-Sn(10) | Mg-Sr(10) | Mg-Y(10) | Mg-Zn(10) | Mg-Zr(10) |
| Mn-Nd(5) | Mn-Ni(5) | Mn-Sc(10) | Mn-Si(10) | Mn-Sm(10) | Mn-Sn(10) | Mn-Sr(10) |
| Mn-Y(10) | Mn-Zn(10) | Mn-Zr(10) | Nd-Ni(10) | Nd-Y(10) | Nd-Zn(10) | Ni-Sb(10) |
| Ni-Sc(10) | Ni-Si(10) | Ni-Sm(10) | Ni-Sn(10) | Ni-Sr(10) | Ni-Y(10) | Ni-Zn(10) |
| Ni-Zr(10) | O-Si(10) | P-Si(10) | Pr-Sb(10) | Sb-Si(10) | Sb-Sm(10) | Sb-Sn(10) |
| Sb-Zn(10) | Sc-Si(10) | Sc-Sn(10) | Sc-Sr(10) | Sc-Y(10) | Sc-Zn(10) | Sc-Zr(10) |
| Si-Sn(10) | Si-Sr(10) | Si-Y(5) | Si-Zn(5) | Si-Zr(10) | Sm-Sn(10) | Sm-Zn(10) |
| Sn-Sr(10) | Sn-Y(10) | Sn-Zn(10) | Sn-Zr(5) | Sr-Y(5) | Sr-Zn(10) | Y-Zn(10) |
| Y-Zr(10) | Zn-Zr(10) |
Ternary Systems (114)
| Ag-Al-Cu(10) | Ag-Al-Mg(5) | Ag-Cu-Mg(5) | Ag-Gd-Mg(10) | Ag-Mg-Nd(10) | Ag-Mg-Sb(5) |
| Al-Bi-Mg(10) | Al-C-Mg(10) | Al-C-Si(10) | Al-Ca-Ce(5) | Al-Ca-Fe(5) | Al-Ca-Li(5) |
| Al-Ca-Mg(10) | Al-Ca-Si(5) | Al-Ca-Sr(10) | Al-Ce-Gd(5) | Al-Ce-La(5) | Al-Ce-Mg(10) |
| Al-Ce-Mn(5) | Al-Ce-Nd(5) | Al-Ce-Si(10) | Al-Ce-Y(5) | Al-Cu-Gd(5) | Al-Cu-Li(10) |
| Al-Cu-Mg(10) | Al-Cu-Mn(10) | Al-Cu-Nd(5) | Al-Cu-Si(10) | Al-Cu-Sn(10) | Al-Cu-Zn(10) |
| Al-Fe-Mn(10) | Al-Fe-Si(10) | Al-Gd-La(5) | Al-Gd-Mg(10) | Al-Gd-Mn(5) | Al-Gd-Nd(5) |
| Al-Gd-Y(5) | Al-La-Nd(5) | Al-La-Y(5) | Al-Li-Mg(10) | Al-Li-Mn(5) | Al-Li-Si(10) |
| Al-Mg-Mn(10) | Al-Mg-Sc(10) | Al-Mg-Si(10) | Al-Mg-Sn(10) | Al-Mg-Sr(10) | Al-Mg-Y(5) |
| Al-Mg-Zn(10) | Al-Mn-Nd(5) | Al-Mn-Sc(5) | Al-Mn-Si(10) | Al-Nd-Y(5) | Al-Si-Y(5) |
| Al-Si-Zn(5) | Al-Sn-Zn(10) | Bi-Mg-Sn(10) | C-Li-Si(10) | C-Li-Sn(5) | Ca-Ce-Mg(10) |
| Ca-Fe-Si(10) | Ca-Li-Mg(10) | Ca-Li-Si(10) | Ca-Mg-O(10) | Ca-Mg-Si(10) | Ca-Mg-Sn(10) |
| Ca-Mg-Sr(10) | Ca-Mg-Zn(10) | Ca-Sr-Zn(5) | Ce-La-Mg(10) | Ce-Li-Mn(5) | Ce-Mg-Mn(5) |
| Ce-Mg-Nd(10) | Ce-Mg-Sn(10) | Ce-Mg-Y(10) | Ce-Mg-Zn(10) | Cu-Fe-Si(10) | Cu-La-Ni(10) |
| Cu-Li-Mg(10) | Cu-Mg-Ni (10) | Cu-Mg-Si(10) | Cu-Mg-Y(10) | Cu-Mg-Zn(10) | Cu-Ni-Si(5) |
| Cu-Sn-Zn (5) | Cu-Y-Zr(10) | Fe-Mg-Si (10) | Fe-Mn-Si(10) | Gd-Li-Mg(10) | Gd-Mg-Mn(5) |
| Gd-Mg-Y(10) | Gd-Mg-Zn(10) | La-Mg-Nd(10) | La-Mg-Si(5) | La-Mg-Zn(10) | Li-Mg-Mn(5) |
| Li-Mg-Si(10) | Li-Mg-Zn(10) | Li-Si-Sn(5) | Mg-Mn-Sc(5) | Mg-Mn-Si(5) | Mg-Mn-Sr(10) |
| Mg-Mn-Y(5) | Mg-Mn-Zn(10) | Mg-Mn-Zr(5) | Mg-Nd-Y(5) | Mg-Nd-Zn(5) | Mg-Ni-Si(5) |
| Mg-Si-Sn(10) | Mg-Si-Zn(5) | Mg-Sn-Zn(10) | Mg-Y-Zn(10) | Mg-Y-Zr(5) | Mn-Y-Zr(10) |
Quaternary Systems (17)
| Ag-Al-Cu-Mg(5) | Al-Bi-Mg-Sn(10) | Al-Ca-Ce-Mg(5) | Al-Ca-Li-Mg(5) | Al-Ca-Mg-Mn(9) |
| Al-Ce-Li-Mg(5) | Al-Cu-Mg-Si(10) | Al-Cu-Mg-Zn(10) | Al-Li-Mg-Si(10) | Al-Mg-Mn-Zn(10) |
| Ca-Ce-Mg-Sn(10) | Ca-Mg-Si-Sn (10) | Ce-Gd-Mg-Y(5) | Gd-Mg-Mn-Sc(5) | Gd-Mg-Y-Zn(9) |
| Mg-Mn-Sc-Y(5) | Mg-Mn-Y-Zr(5) |
Database Validation
The early development of the Mg-database, starting in 1995, is described in [2001Sch] and aspects of quality assurance were first given by [2005Sch].
Progress in systematic development and applications of the current thermodynamic database for Mg alloys is reported in detail in [2018Sch] and [2019Sch]. Models for multicomponent alloys are built in a methodical approach from quantitative descriptions of unary, binary and ternary subsystems [2012Sch]. For a large number of ternary- and some higher- alloy systems, an evaluation of the modeling depth is made with concise reference to experimental work validating these thermodynamic descriptions. A special focus is on ternary intermetallic phase compositions. These comprise solutions of the third component in a binary compound as well as truly ternary solid solution phases, in addition to the simple ternary stoichiometric phases. Concise information on the stability ranges is given. That evaluation is extended to selected quaternary and even higher alloy systems. Thermodynamic descriptions of intermetallic solution phases guided by their crystal structure are also elaborated and the diversity of such unified phases is emphasized [2012Sch].
Key issues in this large thermodynamic Mg alloy database are consistency, coherency and quality assurance. These issues and the basic concept are elaborated in [2013Gro]. The structurally supported modeling, especially of pertinent solid phases, requires proper consideration of multicomponent solid solutions of intermetallic phases which are abundant in magnesium alloys. Moreover, evidence on the database application by predicting phase formation during solidification or heat treatment in advanced multicomponent magnesium alloys from thermodynamic calculations is given in detail in [2013Gro]. Highlights of the most recent progress are given in [2015Sch1].
The growing modeling depth of the PanMagnesium database enhances predictive type calculations of phase formation during solidification, heat treatment or other processing steps of Mg alloys. Evidence is given by comparing the results of such calculations with the phase formation reported in studies of advanced magnesium alloys, such as Mg-Zn-Y/Zr/Gd/Ce/Nd, Mg-Al-Ca/Mn/Sr/Sn, Mg-Sn-Ca and higher order Mg-Al-Sn-Ca/Sr and Mg-Sn-Ca-Ce/Gd/Zr alloys [2013Gro]. Reference is made to the extensive material provided in that most current publication which is not repeated here.
The current thermodynamic database for magnesium alloys has also been extensively tested and validated using published experimental data [1998Lia, 1999Gro, 2001Gro4, 2001Gro6, 2001Kev1, 2001Kev2, 2001Kev3, 2002Gro1, 2002Gro2, 2003Gro1, 2003Gro2, 2004Bru, 2004Kev, 2004Gro, 2006Ohn4]. Some sub-quaternary systems of this database have been critically assessed: Mg-Al-Ca-Ce, Mg-Al-Ca-Li, Mg-Al-Ce-Li, Mg-Al-Cu-Zn, Mg-Mn-Y-Zr. The quaternary systems Mg-Al-Li-Si [2001Kev4] and Mg-Ce-Mn-Sc, Mg-Gd-Mn-Sc, Mg-Mn-Sc-Y [2000Pis2, 2001Gro2] were thermodynamically modeled and used for technical applications.
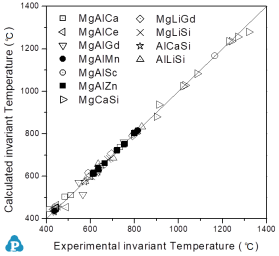
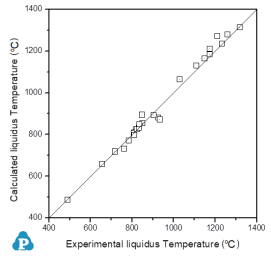
For the reliability of the calculated phase diagrams the fitting of the invariant temperatures are of paramount importance. Since the measured temperatures of the invariant reactions are not affected by super-cooling related problems, these nonvariant data are perfect criteria for comparison of experimental with calculated data (as shown in Figure 1).
Figure 1: Invariant temperatures for various ternary alloys included in PanMagnesium: Comparison between calculated and experimental data
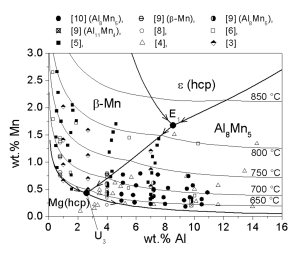
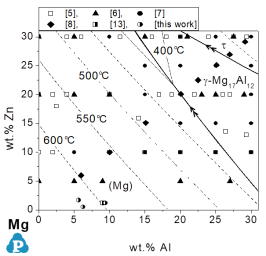
For the Mg-Al-Mn system the reliability is checked in detail [2005Ohn]. The liquidus surface of the Mg-rich corner is shown in Figure 2. The same experimental data are plotted in Figure 3 as comparison between calculated results and experimental data.
Figure 2: Calculated partial liquidus surface, the thick lines indicate monovariant reaction lines and the thin lines represents the isotherms. Superimposed are the compositions of experimental alloys further compared to calculated data of the liquidus surface. The primary solid phase is specified in some experimental data
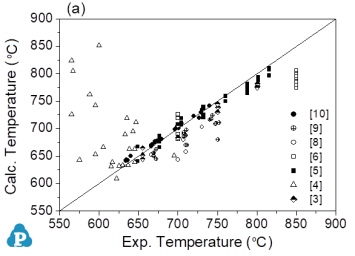
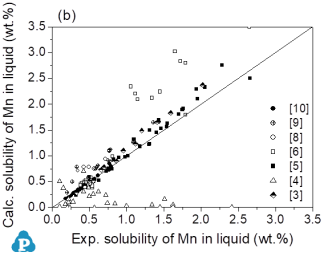
This comparison in Figure 3 enables easy identification of those groups of experimental data that are not consistent with the bulk of experimental work. There is a reasonable agreement with this bulk of experimental data and the calculated values. Moreover, there is a reasonable agreement with the primary solidifying phases as shown in Figure 2.
Figure 3: Comparison between calculated results and experimental data for all alloy samples in the Mg-Al-Mn system. (a) Liquidus temperature at a given composition, (b) Solubility of Mn in liquid at a given temperature and Al composition. The straight line in Figs. (a) and (b) is a visual aid corresponding to perfect agreement between experimental values and the calculated results from the present thermodynamic model
The experimental data for the commercially very important Mg-Al-Zn alloys are shown in Figure 4 [2006Ohn1]. The liquidus surface of the Mg-rich corner is given inFigure 4. The same experimental data is plotted in Figure 5 as comparison between calculated results and experimental data. A similar comparison was done for the Mg-rich phase equilibria of the Mg-Mn-Zn system [2006Ohn2]. Technical important liquidus and Solidus temperatures of Mg-rich Mg-Al-Mn-Zn Alloys (AZ series) were investigated by [2006Ohn3].
Figure 4: Calculated partial Mg-Al-Zn liquidus surface and experimental alloy compositions. The thick lines indicate monovariant reaction lines and the dashed lines represent isotherms at an interval of 50°C
Figure 5: Comparison between calculated and experimental liquidus temperature for all alloy samples in the Mg-Al-Zn system. The straight line is a visual aid corresponding to perfect agreement between experimental and calculated results
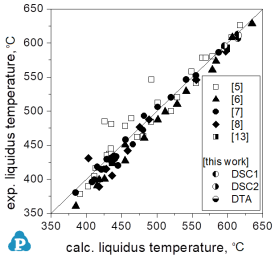
Measured liquidus temperatures for other ternary Mg-alloy systems are compared in Figure 6 with calculations from the magnesium database. These miscellaneous Mg-X1-X2 systems include the alloying elements Al, Ca, Ce, Gd, Li, Sc, and Si.
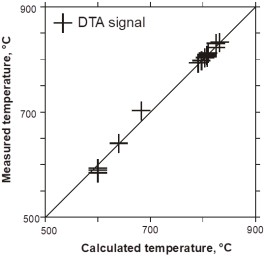
Additionally, a selected comparison of experimental data and calculated phase equilibria is given below. This demonstrates the feasibility to perform reasonable calculations with PanMagnesium even in some very high alloyed regions with vanishing Mg-content as outlined in Figure 6. For the Al-Li-Si system a comparison between calculated and experimentally measured DTA data is given in Figure 7 [2001Gro6]. Figure 8 shows a calculated vertical section in the Al-Ce-Si system at constant 90at% Al including the DSC/DTA signals measured [2004Gro].
Figure 6: Liquidus temperatures for various ternary magnesium alloys outside the Mg-Al-Mn-Zn system, with alloying elements Al, Ca, Ce, Gd, Li, Sc, Si: Comparison between calculated and experimental data
Figure 7: Comparison between calculated and experimentally measured DTA data for the Al-Li-Si system
Figure 8: Calculated vertical section Al90Ce10 - Al90Si10 at constant 90at%Al including the DSC/DTA signals measured in [2004Gro]
Advanced Mg alloys employing Long Period Stacking Order (LPSO) and icosahedral precipitate phases for superior properties have been studied in more depth recently. For the most important ternary alloy systems Mg-Y-Zn and Mg-Gd-Zn, these findings are used to advance the description in PanMg beyond the previous knowledge [2015Sch2, 2015Gro].
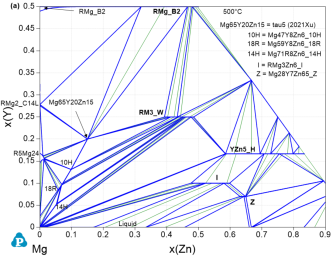
Figure 9: Calculated Mg-Y-Zn isothermal section at 500°C; (a) up to 50 at.% Y and 90 at.% Zn; (b) up to 16 at.% Y and 12 at.% Zn compared with the key alloys of [2023Rua]
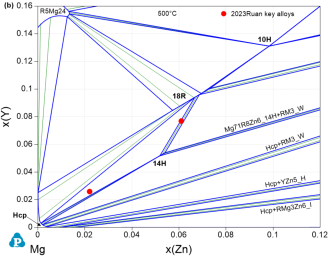
That is exemplified for Mg-Y-Zn in the calculated isothermal sections at 500°C shown for Mg-rich corner in Figure 9. The detail in Figure 9b shows the perfect agreement with the dedicated TEM study of the two key alloys of [2023Rua] in the 14H+18R two-phase and (Mg)+14H+18R three-phase regions. This also reflects the experimentally verified solid solution ranges of these two LPSO phases 14H and 18R in the TEM studies [2021Rua1, 2021Rua2], confirmed by the experimental data indicated in [2015Sch2], that were simplified to stoichiometric phases at that time in PanMg2015 and the previous study [2012Gro]. The phase equilibria at lower Mg-content in Figure 9a are also confirmed by the mutually consistent experimental data assessed from [2012Gro, 2015Sch2, 2021Xu1] and include the new compound Mg65Y10Zn15 [2021Xu1].
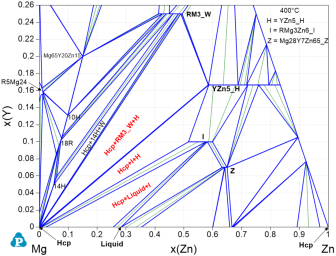
Figure 10: Calculated Mg-Y-Zn isothermal section at 400°C up to 26 at.% Y and 100 at.% Zn; three-phase regions (Mg)+Liquid+I, (Mg)+I+H, and (Mg)+RM3_W verified by the key alloys of [2020Liu] are marked red.
At 400°C the equilibria (Mg)+Liquid+I, as opposed to (Mg)+Liquid+Z, as well as (Mg)+I+H and (Mg)+RM3_W, confirmed by TEM [2020Liu], are exactly represented in the Mg-Y-Zn isothermal section of Figure 10 calculated with PanMg2024. None of the currently published alternative CALPHAD descriptions, e.g. [2021Xu1, 2023Rua], is capable of describing the entity of experimentally verified data of the Mg-Y-Zn system as shown by PanMg2024.
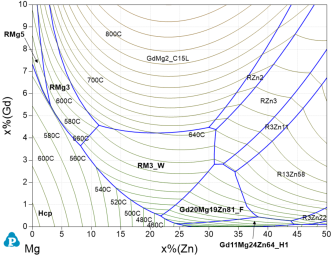
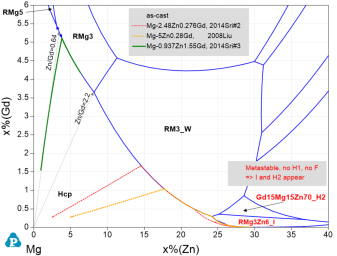
The Mg-Gd-Zn system includes the solid-solution LPSO phase 14H and the re-optimization is validated by the experimental data of the entire stable phase equilibria [2012Qi, 2015Gro] as well as the intricate metastable phases I and H2 [2016Gro]. The experimental fact that after heat treatment at 400 or 500°C, the I and the H2 phases, observed in cast alloys, transform into the stable phases F and H1 [2016Gro] are reflected in the Mg-Gd-Zn isothermal sections at 400 and 500°C for the stable system and the metastable system with suspended phases F and H1, calculated with PanMg2024. In addition, the more recent experimental data [2023Xu, 2021Xu2, 2021Liu] were considered but found less conclusive. The as-cast formation conditions of the icosahedral I phase, modeled as RMg3Zn6_I with R = (Y,Gd) solubility, is detailed in the calculated Mg-Gd-Zn liquidus projections for the stable system, Figure 11, and the metastable system, Figure 12, where only a small supercooling leads to the crystallization fields of I and H2 phases. These data are, in addition to [2016Gro], validated by the experimental studies of as-cast [2008Liu, 2014Sri, 2016Li, 2018Luo] and directionally solidified [2017Yan] alloys. As example, the Scheil-simulated solidification paths superimposed on Figure 12 for the two alloys with high Zn/Gd ratio [2014Sri#2, 2008Liu] demonstrate after primary (Mg) the secondary phases RM3_W and RMg3Zn6_I as also observed experimentally. By contrast, the alloy with low Zn/Gd ratio [2014Sri#3] show after primary (Mg) only the secondary phase RMg3 (with 23 at.% Zn) at the Scheil-solidus of 555°C. For that alloy the equilibrium calculation indicates the formation of 14H in the reaction RMg3 + (Mg) = 14H at about 536°C. That is well verified by the observed microstructure where the small fraction of LPSO phase next to RMg3 in alloy #3 was assumed to be formed due to the relatively slow cooling rate [2014Sri].
Figure 11: Calculated Mg-Gd-Zn liquidus projection up to 10 at.% Gd and 50 at.% Zn include the stable phases F and H1.
Figure 12: Calculated Mg-Gd-Zn metastable liquidus projection up to 6 at.% Gd and 40 at.% Zn with phases F and H1 suspended, thus, the next stable phases I and H2 appear. Scheil-simulated solidification paths of three alloys are superimposed, see text.
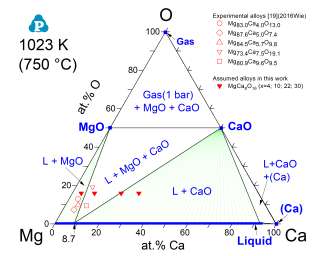
Another highlight of PanMg is the incorporation of oxygen as an element and all relevant solid oxides and gas species. For example, the reaction of Mg with CaO during melting and solidification was studied experimentally and the thermodynamic description of the Mg-Ca-O system was developed with scrutinized oxide data. The calculated isothermal section in Figure 13 is also validated using in-situ synchrotron radiation diffraction [2018Lia1, 2018Lia2].
Figure 13: Calculated isothermal section of the Mg-Ca-O system, validated using in-situ synchrotron radiation diffraction [2018Lia1]
The good agreement between experimental and calculated results, as shown in all of the above figures indicates the reliability of the current PanMagnesium thermodynamic database. Additional validation is given in some comprehensive studies [2012Sch, 2013Gro]. Applications are highlighted in [2018Sch].
[1991Din] A.T. Dinsdale, SGTE data for pure elements, Calphad, 15 (1991) 317-425. doi:10.1016/0364-5916(91)90030-N.
[1995She] H. Shen, W. Zhang, G. Liu, R. Wang, Z. Du, Thermodynamic Evaluation of Yttrium, Rare Met. (China), 14 (1995) 193-202.
[1996She] H. Shen, W. Zhang, G. Liu, R. Wang, Z. Du, Thermodynamic Evaluation of Gadolinium, Rare Met. (China), 15 (1996) 23-34.
[1998Lia] Liang, P., Tarfa, T., Robinson, J.A., Wagner, S., Ochin, P., Harmelin, M.G., Seifert, H.J., Lukas, H.L., Aldinger, F., Experimental investigation and thermodynamic calculation of the Al-Mg-Zn system, Thermochim. Acta, 314 (1998): 87-110.
[1999Gro] Gröbner, J., Schmid-Fetzer, R., Pisch, A., Cacciamani, G., Riani, P., Ferro, R.: Experimental Investigations and Thermodynamic Calculation in the Al-Mg-Sc System. Z. Metallkd. 90(11) (1999): 872-880.
[2000Pis2] Pisch, A., Gröbner, J., Schmid-Fetzer, R.: Application of Computational Thermochemistry to Al- and Mg-alloy processing with Sc additions. Mat. Sci. Eng. A 289 (2000): 123-129.
[2001Gro2] Gröbner, J., Pisch, A., Schmid-Fetzer, R.: Selection of Promising Quaternary Candidates from Mg Mn (Sc, Gd, Y, Zr) for Development of Creep resistant Magnesium Alloys. J. Alloys Comp. 320/2, (2001): 296-301.
[2001Gro4] Gröbner, J., Kevorkov, D., Schmid-Fetzer, R.: Thermodynamic Calculation of Al-Gd and Al-Gd-Mg Phase Equilibria Checked by Key Experiments. Z. Metallkd. 92 (2001): 22-27.
[2001Gro6] Gröbner, J., Kevorkov, D., Schmid-Fetzer, R.: The Al-Li-Si System, Part 2: Experimental study and Thermodynamic Calculation of the polythermal equilibria. J. Solid State Chem. 156 (2001): 506-511.
[2001Kev1] Kevorkov, D., Gröbner, J., Schmid-Fetzer, R.: The Al-Li-Si system, Part 1: A new structure type Li8Al3Si5 and the ternary solid state phase equilibria. J. Solid State Chem. 156 (2001): 500-505.
[2001Kev2] Kevorkov, D.G., Pavlyuk, V.V., Dmytriv, G.S., Bodak, O.I., Gröbner, J., Schmid-Fetzer, R.: The ternary Gd-Li-Mg system: Phase diagram study and computational evaluation. J. Phase Equilibria 22 (1) (2001): 34-42.
[2001Kev3] Kevorkov, D., Schmid-Fetzer, R.: The Al-Ca System Part 1: Experimental Investigation of Phase Equilibria and Crystal Structures. Z. Metallkd. 92 (2001): 946-952.
[2001Kev4] Kevorkov, D.: Thermodynamics and Phase Equilibria of the Mg-Al-Li-Si System, Ph. D. Thesis TU Clausthal 2001.
[2001Sch] Schmid-Fetzer, R., Gröbner, J.: Focused Development of Magnesium Alloys Using the Calphad Approach. Advanced Engineering Materials 3 (12) (2001): 947-961.
[2001Cel] S. Celotto and T.J. Bastow, Study of precipitation in aged binary Mg–Al and ternary Mg–Al–Zn alloys using 27Al NMR spectroscopy. Acta Materialia, 49(1) (2001): 41-51.
[2002Gro1] Gröbner, J., Schmid-Fetzer, R., Pisch, A., Colinet, C., Pavlyuk, V.V., Dmytriv, G.S., Kevorkov, D.G., Bodak, O.I: Phase equilibria, calorimetric study and thermodynamic modelling of Mg-Li-Ca alloys, Thermochimica Acta 389 (2002): 85-94.
[2002Gro2] Gröbner, J., Schmid-Fetzer, R.:Thermodynamic Modeling of Al-Ce-Mg Phase Equilibria Coupled with Key Experiments, Intermetallics 10 (2002): 415-422.
[2002Cac] C.H. Caceres, Hardness and yield strength in cast Mg-Al alloys. AFS Transactions, 110 (2002): 1163-1169.
[2003Gro1] Gröbner, J., Kevorkov, D., Chumak, I. and Schmid-Fetzer, R.: Experimental Investigation and Thermodynamic Calculation of Ternary Al-Ca-Mg Phase Equilibria, Z. Metallkd. 94 (2003): 976-982.
[2003Gro2] Gröbner, J., Kevorkov, D.and Schmid-Fetzer, R.: A new Thermodynamic data set for the binary System Ca-Si and Experimental Investigation in the ternary System Ca-Mg-Si, Intermetallics 11 (2003): 1065-1074.
[2004Bru] Brubaker, C.O., Liu, Z.-K., A computational thermodynamic model of the Ca–Mg–Zn system, J. Alloys Comp. 370 (2004): 114-122.
[2004Gro] J. Gröbner, D. Mirkovic, Rainer Schmid-Fetzer: Thermodynamic Aspects of Constitution, Grain Refining and Solidification Enthalpies of Al-Ce-Si Alloys. Metall. Mater. Trans. A, 35 (2004), 3349-3362.
[2004Kev] Kevorkov, D., Schmid-Fetzer, R. and Zhang F.: Phase Equilibria and Thermodynamics of the Mg-Si-Li System and Remodeling of the Mg-Si System, J. Phase Equil. Diff., 25 (2004): 140-151.
[2005Ohn] M. Ohno, R. Schmid-Fetzer: Thermodynamic assessment of Mg-Al-Mn phase equilibria, focusing Mg-rich alloys. Z. Metallkde. 96 (2005): 857-869.
[2005Sch] R. Schmid-Fetzer, A. Janz, J. Gröbner and M. Ohno: Aspects of quality assurance in a thermodynamic Mg alloy database. Advanced Engineering Materials, 7 (2005): 1142-1149.
[2006Ohn1] M. Ohno, D. Mirkovic and R. Schmid-Fetzer: Phase equilibria and solidification of Mg-rich Mg-Al-Zn alloys. Materials Science & Engineering A, 421 (2006): 328-337.
[2006Ohn2] M. Ohno and R. Schmid-Fetzer: Mg-rich phase equilibria of Mg-Mn-Zn alloys analyzed by computational thermochemistry. Int. J. Materials Research (Z. Metallkunde) 97 (2006): 526-532.
[2006Ohn3] M. Ohno, D. Mirkovic and R. Schmid-Fetzer: Liquidus and Solidus Temperatures of Mg-rich Mg-Al-Mn-Zn Alloys. Acta Materialia, 54 (2006): 3883–3891.
[2006Ohn4] M. Ohno, A. Kozlov, R. Arroyave, Z.K. Liu, and R. Schmid-Fetzer: Thermodynamic modeling of the Ca-Sn system based on finite temperature quantities from first-principles and experiment. Acta Materialia, 54 (2006): 4939-4951.
[2008Liu] Y. Liu, G.Y. Yuan, S. Zhang, X.P. Zhang, C. Lu, W.J. Ding, Effects of Zn/Gd ratio and content of Zn, Gd on phase constitutions of Mg alloys, Materials Transactions 49(5) (2008) 941-944.
[2011Cao] W. Cao, et al., An Integrated Computational Tool for Precipitation Simulation. JOM, 63(7) (2011): 29-34.
[2012Gro] Gröbner, J., Kozlov, A., Fang, X. Y., Geng, J., Nie, J. F. and Schmid-Fetzer, R., "Phase equilibria and transformations in ternary Mg-rich Mg–Y– Zn alloys." Acta Mater. (2012) 60(17): 5948-5962.
[2012Qi] H.Y. Qi, G.X. Huang, H. Bo, G.L. Xu, L.B. Liu, Z.P. Jin, Experimental investigation and thermodynamic assessment of the Mg–Zn–Gd system focused on Mg-rich corner, J. Mater. Sci. 47(3) (2012) 1319-1330.
[2012Sch] R. Schmid-Fetzer, J. Gröbner: Thermodynamic database for Mg alloys — progress in multicomponent modeling. Metals, 2, 377-398 (2012). (Special Issue "Magnesium Technology"), Open Access, https://www.mdpi.com/2075-4701/2/3/377
[2013Gro] J. Gröbner, R. Schmid-Fetzer: Key issues in a thermodynamic Mg alloy database. Metall. Mater. Trans. A, A44, 2918-2934 (2013) dx.doi.org/10.1007/s11661-012-1483-z
[2013Pal] M. Paliwal, Microstructural development in Mg alloys during solidification: an experimental and modeling study, in Department of Mining and Materials Engineering 2013, McGill University: Montreal, QC.
[2013Pal2] M. Paliwal and I.-H. Jung, The evolution of the growth morphology in Mg–Al alloys depending on the cooling rate during solidification. Acta Materialia, 61(13) (2013): 4848-4860.
[2014Hof] Hofstetter, J., Becker, M., Martinelli, E., Weinberg, A. M., Mingler, B., Kilian, H., Pogatscher, S., Uggowitzer, P. J. and Löffler, J. F., "High-Strength Low-Alloy (HSLA) Mg–Zn–Ca Alloys with Excellent Biodegradation Performance" JOM (2014) 566-572. 66(4) doi:10.1007/s11837-014-0875-5
[2014Kam] C.C. Kammerer, et al., Interdiffusion and impurity diffusionin polycrystalline Mg solid solution with Al or Zn. Journal of Alloys and Compounds, 617 (2014): 968-974.
[2014Sri] A. Srinivasan, Y. Huang, C.L. Mendis, C. Blawert, K.U. Kainer, N. Hort, Investigations on microstructures, mechanical and corrosion properties of Mg–Gd–Zn alloys, Materials Science and Engineering: A 595 (2014) 224-234.
[2015Gro] J. Gröbner, A. Kozlov, X.Y. Fang, S.M. Zhu, J.F. Nie, M.A. Gibson, R. Schmid-Fetzer: Phase equilibria and transformations in ternary Mg–Gd–Zn alloys.Acta Materialia, 90 (2015): 400–416.
[2015Sch1] R. Schmid-Fetzer: Progress in thermodynamic database development for ICME of Mg alloys, in Magnesium Technology 2015, Edited by: M.V. Manuel, A. Singh, M. Alderman, and N.R. Neelameggham,TMS (The Minerals, Metals & Materials Society), 283-287 (2015)
[2015Sch2] Rainer Schmid-Fetzer, Joachim Gröbner, Michiaki Yamasaki, Yoshihito Kawamura, Hiroshi Okuda, Seiji Miura, Toshiaki Horiuchi, Jian-Feng Nie: Thermal stability of Mg-Zn-Y LPSO phases and revised thermodynamic description of the Mg-Zn-Y phase diagram. Mg 2015 - The 10th International Conference on Magnesium Alloys and Their Applications. October 11-16, 2015, Jeju, Korea.Symp. LPSO Structure & Its Related Alloys, Invited, October 12, 2015. eProceedings, EO-3-0372, pp 1-23.
[2016Cao] J.D. Cao, T. Weber, R. Schäublin, J.F. Löffler, Equilibrium ternary intermetallic phase in the Mg-Zn-Ca system, J. Mater. Res., 31 (2016) 2147-2155. doi:10.1557/jmr.2016.196.
[2016Gro] J. Gröbner, S. Zhu, J.-F. Nie, M.A. Gibson, R. Schmid-Fetzer, Metastable phase formation in ternary Mg–Gd–Zn alloys, J. Alloys and Compounds 675 (2016) 149-157.
[2016Li] J. Li, Z. He, P. Fu, Y. Wu, L. Peng, W. Ding, Heat treatment and mechanical properties of a high-strength cast Mg–Gd–Zn alloy, Materials Science and Engineering: A 651 (2016) 745-752.
[2016Kam] C.C. Kammerer, et al., Interdiffusion in Ternary Magnesium Solid Solutions of Aluminum and Zinc. Journal of Phase Equilibria and Diffusion, 37(1) (2016): 65-74.
[2017Yan] G. Yang, S. Luo, S. Liu, L. Xiao, W. Jie, Microstructural evolution, phase constitution and mechanical properties of directionally solidified Mg-5.5Zn-xGd (x = 0.8, 2.0, and 4.0) alloys, J. Alloys and Compounds 725 (2017) 145-154.
[2018Lia1] S.-M. Liang, A. Kozlov, R. Schmid-Fetzer, The Mg–Ca–O system: Thermodynamic analysis of oxide data and melting/solidification of Mg alloys with added CaO, Int. J. Materials Research (Z. Metallkunde), 109 (2018): 185-200.
[2018Lia2] S.-M. Liang, R. Schmid-Fetzer, Complete thermodynamic description of the Mg-Ca-O phase diagram including the Ca-O, Mg-O and CaO-MgO subsystems, Journal of the European Ceramic Society, 38 (2018): 4768-4785.
[2018Luo] L. Luo, Y. Liu, M. Duan, Phase Formation of Mg-Zn-Gd Alloys on the Mg-rich Corner, Materials 11(8) (2018) 1351.
[2018Sch] R. Schmid-Fetzer, F. Zhang: The light alloy Calphad databases PanAl and PanMg. Calphad, 61 (2018): 246-263.
[2019Sch] R. Schmid-Fetzer, Recent Progress in Development and Applications of Mg Alloy Thermodynamic Database, in: V.V. Joshi, J.B. Jordon, D. Orlov, N.R. Neelameggham (Eds.) Magnesium Technology 2019, TMS Annual Meeting, Springer International Publishing, 2019, 249-255. https://doi.org/10.1007/978-3-030-05789-3_37
[2019Zha] C. Zhang, et al., CALPHAD-Based Modeling and Experimental Validation of Microstructural Evolution and Microsegregation in Magnesium Alloys During Solidification. Journal of Phase Equilibria and Diffusion, 40(4) (2019): 495-507.
[2020Liu] B.-S. Liu, H.-X. Li, Y.-P. Ren, M. Jiang, G.-W. Qin, Phase equilibria of low-Y side in Mg– Zn–Y system at 400 °C, Rare Metals, 39 (2020) 262-269. doi:10.1007/s12598-018-1024-z.
[2020Wu1] X. Wu, C. Li, C. Guo, Z. Du, Thermodynamic re-assessment of the Mg–Y binary system coupling with the precipitation sequence during aging process, Calphad, 71 (2020) 102010. doi:10.1016/j.calphad.2020.102010.
[2020Wu2] X. Wu, C. Li, J. Zheng, Y. Ruan, C. Guo, Z. Du, B. Chen, Y. Wu, Thermodynamic re-assessment of the Mg–Gd binary system coupling the microstructure evolution during ageing process, Calphad, 68 (2020) 101712. https://doi.org/10.1016/j.calphad.2019.101712.
[2021Liu] B. Liu, H. Li, Y. Ren, H. Xie, H. Pan, M. Jiang, G. Qin, Phase relationship in the Mg-rich corner of the Mg-Zn-Gd ternary system at 400–475° C, J. Alloys and Compounds 870 (2021) 159502.
[2021Rua1] Ruan, Y., Li, C., Ren, Y., Wu, X., Schmid-Fetzer, R., Guo, C. and Du, Z., "Phases equilibrated with long-period stacking ordered phases in the Mg-rich corner of the Mg-Y- Zn system." J. Mater. Sci. Technol. (2021) 68: 147-159.
[2021Rua2] Y. Ruan, C. Li, R. Schmid-Fetzer, Y. Ren, C. Guo, Z. Du, The thermodynamic tailoring for Mg86Y8Zn6 alloy with sole long-period stacking ordered structures, J. Alloys and Compounds, 861 (2021) 158509. doi: 10.1016/j.jallcom.2020.158509.
[2021Xu1] Xu, K., Liu, S., Chang, K., Liang, Y., Du, Y. and Jin, Z., "A general thermodynamic model for the long-period stacking ordered phases in magnesium alloys." J. Mg. All. (2021) 9(1): 144-155.
[2021Xu2] H. Xu, H.-L. Chen, P. Wang, T. Zhou, Phase equilibria of the Mg–Gd–Zn system at 500 °C, J. Alloys and Compounds 884 (2021) 161048.
[2022Li] X. Li, S. Liu, D. Huang, Y. Du, Thermodynamic modeling of the Mg–Mn–Zn system based on the refinement of the Mg–Zn and Mn–Zn systems, Calphad, 79 (2022) 102479.
[2022Qiu] W. Qiu, G. Huang, Y. Li, J. Chen, W. Huang, Z. Peng, J. Liang, F. Xia, M. Yao, A. Xiong, Microstructure and properties of Mg–Ca–Zn alloy for thermal energy storage, Vacuum, 203 (2022) 111282. doi:https://doi.org/10.1016/j.vacuum.2022.111282.
[2022Sch] R.E. Schäublin, M. Becker, M. Cihova, S.S.A. Gerstl, D. Deiana, C. Hébert, S. Pogatscher, P.J. Uggowitzer, J.F. Löffler, Precipitation in lean Mg–Zn–Ca alloys, Acta Materialia, 239 (2022) 118223. doi:https://doi.org/10.1016/j.actamat.2022.118223.
[2023Rua] Y. Ruan, S. Xia, C. Li, C. Guo, Z. Du, Experimental study on the microstructure and mechanical properties of the Mg-Y- Zn alloys with LPSO phases tailored by CALPHAD method, J. Alloys and Compounds, (2023) 171414. doi: 10.1016/j.jallcom.2023.171414.
[2023Xu] H. Xu, T. Zhou, P. Wang, W. Yang, K. Liu, Solid-State Phase Equilibria at 320°C of the Mg-Gd-Zn System in the Region of Less Than 50 at.% Gd, JOM 75(8) (2023) 3025-3032.